Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
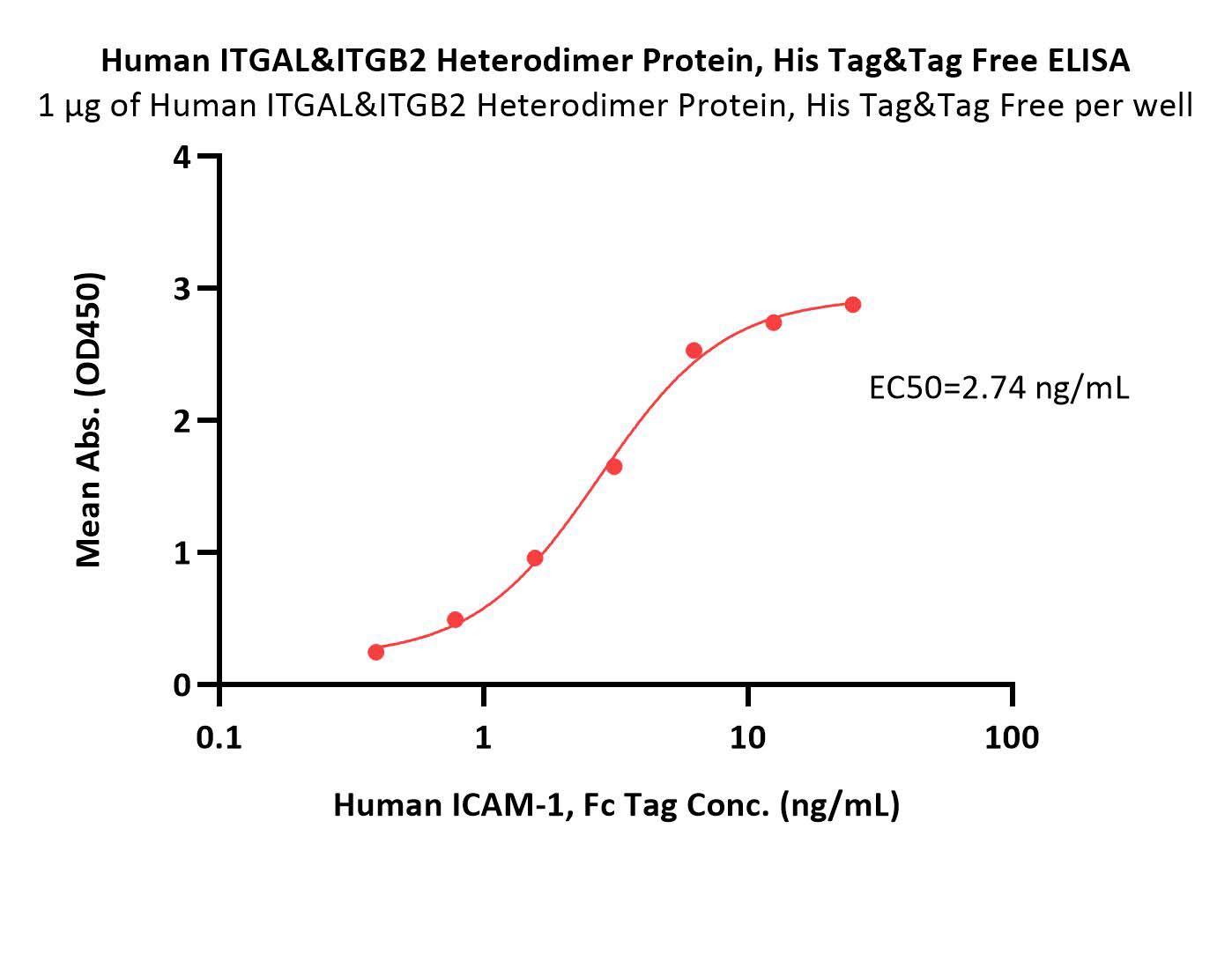
| IT2-H53W3 | Human | Human Integrin alpha L beta 2 (ITGAL&ITGB2) Heterodimer Protein, His Tag&Tag Free |  |

|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Lifitegrast | SHP-606; SAR-1119; SAR-1118; OJL-332; SPD-606 | Approved | Shire Development Llc | Xiidra | United States | Dry Eye Syndromes | Bausch And Lomb Inc | 2016-07-11 | Keratoconjunctivitis Sicca; Graft vs Host Disease; Dry Eye Syndromes; Diabetic macular oedema; Conjunctivitis, Allergic; Xerophthalmia | Details |
| Lifitegrast | SHP-606; SAR-1119; SAR-1118; OJL-332; SPD-606 | Approved | Shire Development Llc | Xiidra | United States | Dry Eye Syndromes | Bausch And Lomb Inc | 2016-07-11 | Keratoconjunctivitis Sicca; Graft vs Host Disease; Dry Eye Syndromes; Diabetic macular oedema; Conjunctivitis, Allergic; Xerophthalmia | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Alintegimod | 7HP349; 7-HP-349; 7HP-349 | Phase 2 Clinical | 7 Hills Pharma LLC | Kidney Neoplasms; Liver Neoplasms; Solid tumours; Mismatch Repair Deficient Cancer; Carcinoma, Renal Cell; Neoplasms; Skin Neoplasms; Mesothelioma; Colorectal Neoplasms; Melanoma; Neoplasm Metastasis; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
| Alintegimod | 7HP349; 7-HP-349; 7HP-349 | Phase 2 Clinical | 7 Hills Pharma LLC | Kidney Neoplasms; Liver Neoplasms; Solid tumours; Mismatch Repair Deficient Cancer; Carcinoma, Renal Cell; Neoplasms; Skin Neoplasms; Mesothelioma; Colorectal Neoplasms; Melanoma; Neoplasm Metastasis; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung | Details |
This web search service is supported by Google Inc.